ALL MATERIAL COPYRIGHT KEVIN SCOTT 2011. LINKS TO THIS SITE ARE WELCOME BUT DO NOT COPY MATERIAL FROM THIS SITE TO ANY OTHER WEBPAGE.
If you find this site useful, please support it by making a donation of $1 to help maintain and develop it. Click on the PAYPAL DONATE button to do this safely. But there is no obligation - please avail yourself of the information and facilities of the site at no charge.

The Standard Weston Cadmium Cell is now obsolete as a formal standard for the definition of the volt although it was employed for this purpose in the USA and internationally, for part of the 20th century. In Europe, the volt was defined as a derived unit from a standard ampere and ohm until 1990 and then, until 1997, the volt was defined in relation to frequency via the Josephson effect. In the laboratory, the Weston Standard Cell has also been eclipsed by voltage reference devices usually based on temperature controlled zener diodes. As a consequence, it appears that these standard cells are no longer manufactured, but can still be obtained as they are progressively retired from laboratory service. As a means of applying a check to laboratory instruments they can still be useful.
On this page, a Standard Weston Cadmium Cell is assessed using the Solartron 7150plus multimeter interfaced to a computer using a National Instruments GPIB-232CT-A converter.
The International Conference on Electrical Standards (London 1908) defined the ideal qualities of standard cells as follows:
(1) The cells must be constructed from high purity materials and be reproducible.
(2) Except when current is flowing there should be no chemical reaction taking place in the cell.
(3) When the cell is thermally cycled it must give the same emf at a given temperature regardless of its thermal history.
(4) When a current is passed through the cell, its emf may change, but it must recover rapidly and completely on cessation of the current.
(1) to (4) above represent an ideal which only the Weston Standard cell approaches.
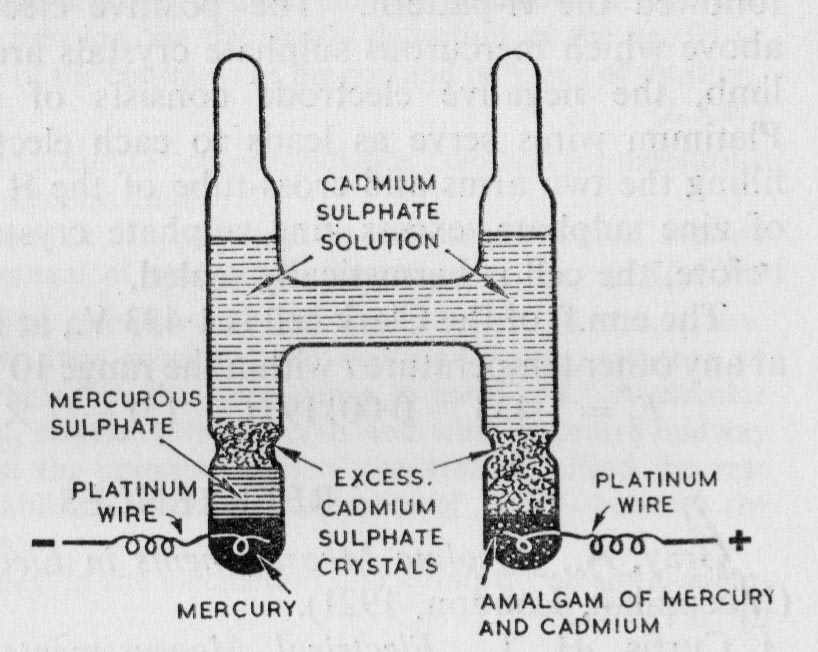
The construction of a Weston Cadmium Cell is shown on the left. In 1908, using an earlier definition of a volt, the cell emf was determined to be 1.0183 volts at 20 degrees C. Subsequently, the international volt was redefined as 1.00033 practical units and so the accepted standard EMF of a Weston Cadmium Cell then became 1.01864 volts, according to the National Physical Laboratory in 1935.
In the original design by Edward Weston in 1892, the electrolyte was unsaturated. This had the advantage of producing a cell with a very low temperature coefficient. The 1908 conference specified a saturated solution and now cells of both types are encountered. For the saturated cell, Fewkes & Yarwood give the emf as 1.01864 -0.0000406(t-20) -9.5x10-7(t-20)2 The EMF of the unsaturated cell appears to be 1.018636 - 0.000005(t-20).

The Solartron 7150plus Multimeter has a maximum input current of 150 picoamps which is low enough to permit its recording of the emf of a Weston Standard Cell for long periods without any harm to the cell.
Using the software described elsewhere on this site, a Solartron 7150plus Multimeter was set to monitor the Weston Cadmium Cell continually. After approximately 24 hours, about 51000 data readings were obtained. This data set was resampled to give 500 data points, and these are plotted below:
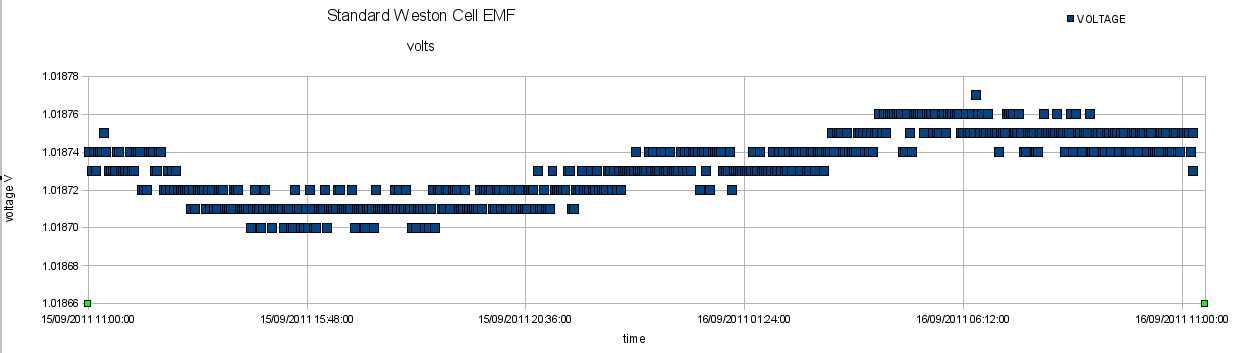
The mean value of the measured EMF was 1.01873 volts with a standard deviation of 0.000018 volts. The accepted value for the Weston Cadmium Cell is 1.01864 volts but this can be subject up to 85 microvolt variation with time. (It appears that the EMF of a cadmium cell can wander about a mean value with 85 microvolts being the maximum deviation. The accuracy of the Solartron Multimeter is 0.002% + 5 digits. Thus the measurements demonstrate that both the multimeter and the standard cell are working within their specified accuracy.