ALL MATERIAL COPYRIGHT KEVIN SCOTT 2011. LINKS TO THIS SITE ARE WELCOME BUT DO NOT COPY MATERIAL FROM THIS SITE TO ANY OTHER WEBPAGE.
If you find this site useful, please support it by making a donation of $1 to help maintain and develop it. Click on the PAYPAL DONATE button to do this safely. But there is no obligation - please avail yourself of the information and facilities of the site at no charge.

Prince Rupert Drops have been described elsewhere as tadpole shaped drops of glass produced by dropping molten glass into cold water. They seem not to have any practical use although, because of small variations in refractive index internally, they sparkle somewhat under bright light. They have, in consequence been incorporated in jewellery. But they remain really a material science curiosity because they self-destruct in a dramatic fashion when tails are broken off. This is evidently the main point of interest.
They evidently came to the attention of Prince Rupert, Duke of Bavaria, Duke of Cumberland in the mid seventeenth century and he brought the Drops probably from Holland and presented them to King Charles II in 1660 who, in turn, gave them to the Royal Society to investigate. Apart from demonstrating the potential energy stored in stressed solids, no useful purpose was found for them.
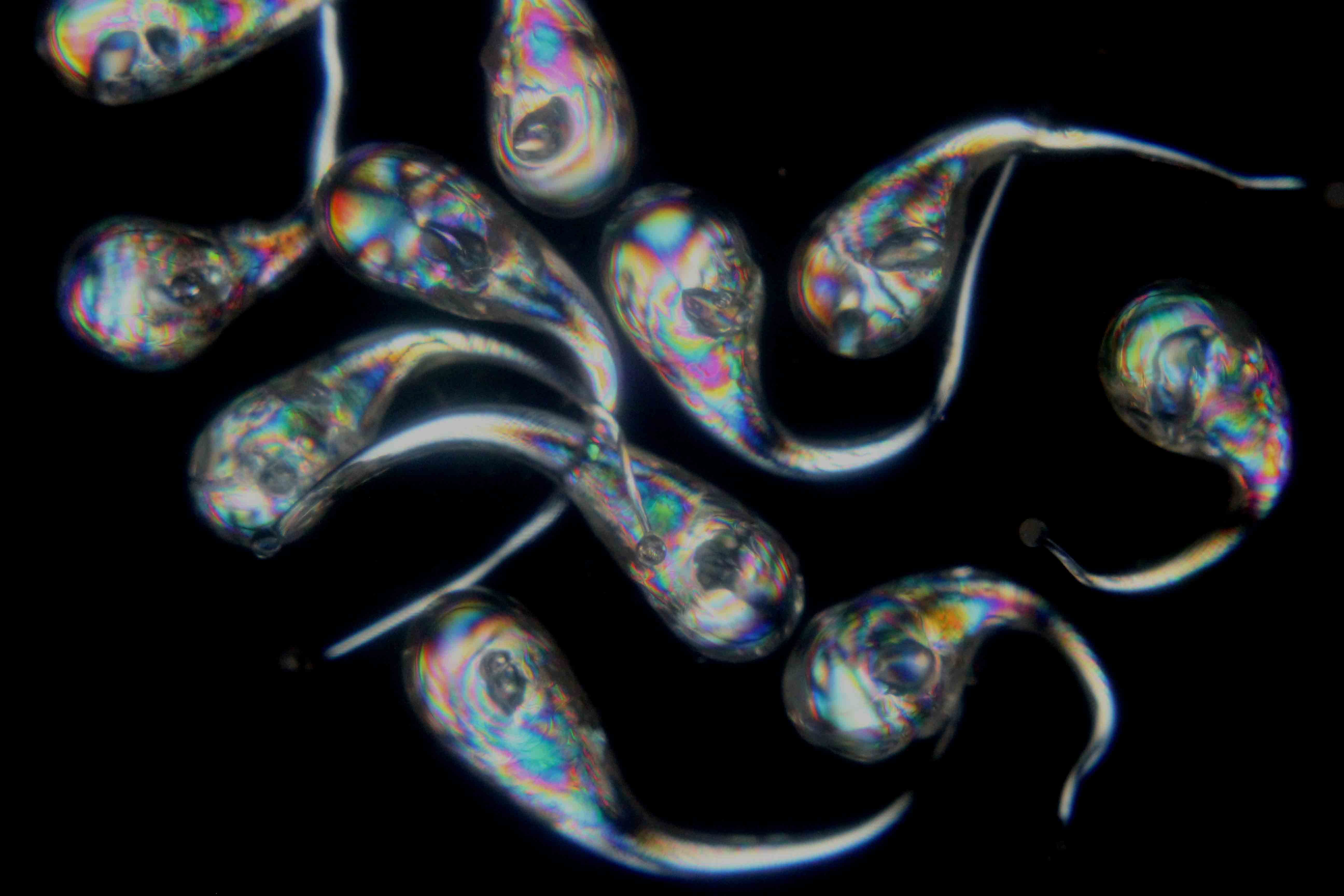
The interest in Prince Rupert Drops lies principally in the fact that they explode when their tails are broken. Otherwise they are quite robust and the larger end is very strong and will resist even quite heavy impact say, from a hammer. Similarly the tail is not easy to break but will do so with the aid of pliers or by means of a sharp impact from a hardened steel pin.The photograph to the right shows the Prince Rupert Drops viewed through a polariscope with the linear polarising elements crossed. The internal stresses in the Drops become clearly visible.
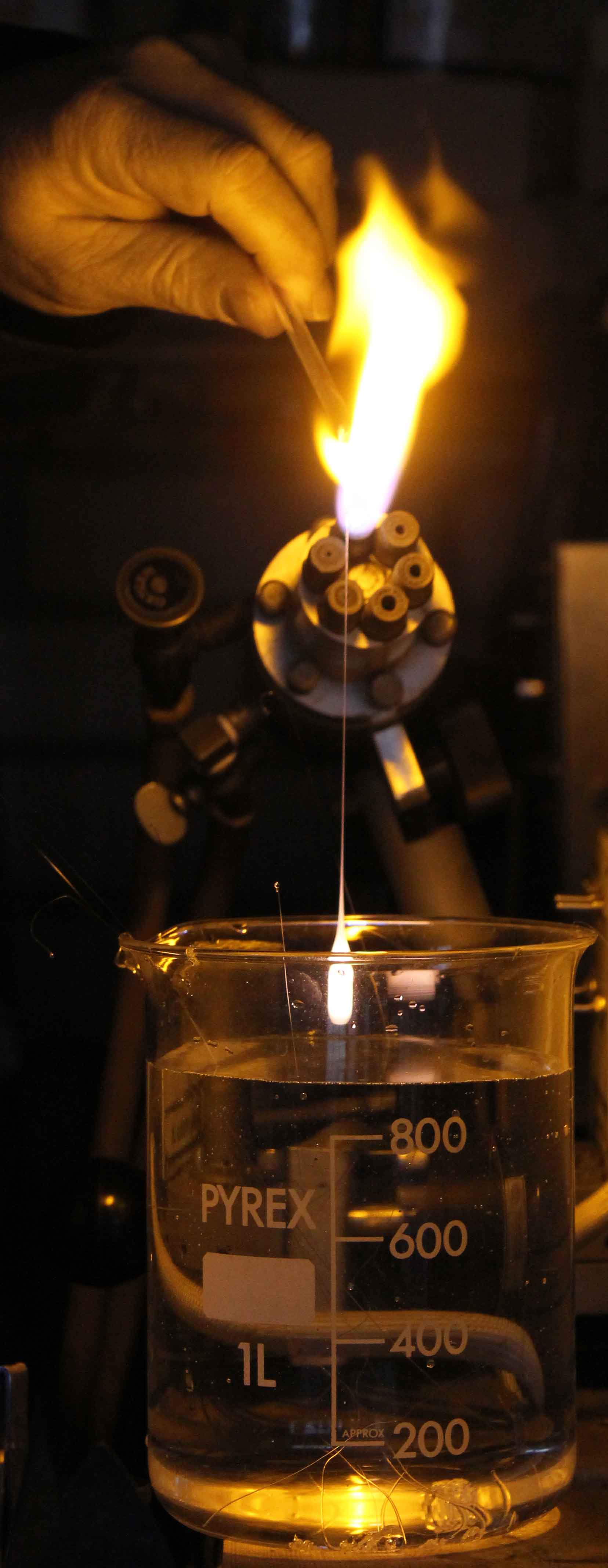
Prince Rupert Drops are very simply made by dropping molten glass into cold water. Those pictured top right were prepared by melting a borosilicate glass rod in an oxy-propane flame and allowing the glass to fall into a 1 litre beaker filled with cold water. The first few drops, disintegrated in the water after a few seconds, but later drops survived as tadpole shapes about 8mm in maximum diameter with a long tapering tail. After retrieving the drops from the water and drying them, the tails could be conveniently shortened in the flame to give a final length of about 20mm.
It was found that soda glass produced drops that shattered on cooling under water, but might survive if quenched in oil rather than water, but this was not tried.
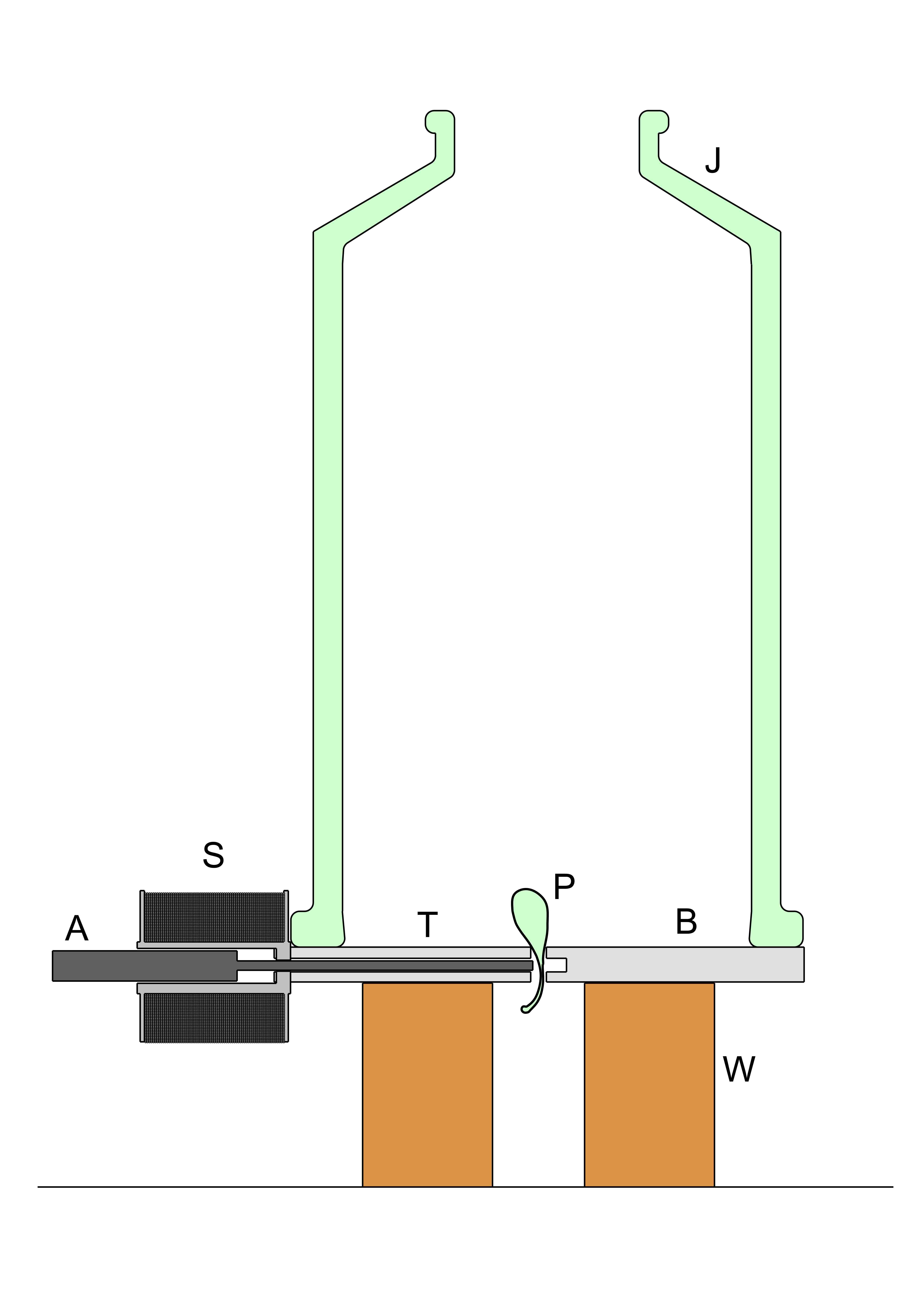
The explosions of Prince Rupert Drops was carried out using the apparatus in the diagram. A steel disk, B, 100 mm in diameter and 5mm thick was drilled to provide a central hole 2.5mm in diameter and a radial hole 1.6mm in diameter. The disk was screwed to the wooden mounting block W. A silver steel pin T 1.6mm in diameter, hardened by quenching in oil from red heat and tempered at 250 °C, was inserted in the horizontal radial hole. It was threaded at the distal end and attached thereby to the soft iron core, A of the solenoid S. A Prince Rupert Drop was inserted tail first into the central aperture in the disk and covered with the bell jar J. A simple relay circuit was arranged so that when a button was pressed, current was supplied to the solenoid and simultaneously the remote shutter release on a Canon EOS 550D camera was actuated. The solenoid armature, A, moved rapidly into the coil causing the hardened pin T to strike the tail of the Prince Rupert Drop and the camera, set at 1/1000 sec exposure time, made an exposure as the Drop exploded into the bell jar.
A simple relay circuit was arranged so that when a button was pressed, current was supplied to the solenoid and simultaneously the remote shutter release on a Canon EOS 550D camera was actuated. The solenoid armature, A, moved rapidly into the coil causing the hardened pin T to strike the tail of the Prince Rupert Drop and the camera, set at 1/1000 sec exposure time, made an exposure as the Drop exploded into the bell jar.
The explosion is a dramatic total disintegration of the Prince Rupert Drop. The whole bell jar filled with shards which are acute-angled and sharp. The typical Prince Rupert Drop made as described here, weighed 0.4 gm and an approximate calculations of the potential energy contained by the stressed drop, suggests an energy density of approximately 2 millijoules/gram glass.This is considered rather too small for any normal calorimetric measurement of the energy released on explosion.