The Restoration of a
Nineteenth Century FitzRoy Barometer.

Admiral FitzRoy has
a deservedly honoured name in the history of barometers and weather
observation and forecasting. Indeed, it may be said that he is the
father of the science of Meteorology as we know it today. His wide
experience as a sea captain and global explorer, his creative intellect
and his keen powers of observation and measurement combined with a
commitment to the welfare of seafarers and fishermen, led him to
realise and exploit the measurement of barometric pressure for the
prediction of storms for the preservation of life and the protection of
shipping.
FitzRoy pioneered
the interpretation of barometric observations and did much to equip sea
ports and fishing harbours with the necessary instruments to make those
interpretations possible. The history of this achievement has been well
recorded by Collins of Barometer
World, (to whose book, FitzRoy and
his barometers,
ISBN-13:978-0-948382-14-7, the interested reader is referred) and it is
not surprising that such a career gave rise to Fitzroy's name being
attached to thousands of barometers. Some such instruments were those
he enabled to be distributed to coastal locations, some were of the
same design and quality, but sold and bought on the open market, and
some were inscribed with FitzRoy's name and carried his "remarks", but
otherwise had little to do with him. Many of these barometers were
manufactured throughout the latter part of the Nineteenth Century and
into the Twentieth, and, being mass-produced, were not always of very
good quality, and certainly not very accurate. Collins points out that
the craftsmen who made them may have had little technical understanding
of what they were making: the length of the barometer tube, and indeed,
the whole instrument can be somewhat too short and the scale
necessarily positioned to give an inaccurate reading.
The instrument which
is the subject of the restoration described here is of such a class. It
looks quite presentable, but the distance from the base of the glass
mercury cistern to the scale is a half of one inch too short, so that
the instrument cannot be properly set up for use at sea level. This
disadvantage was alleviated somewhat by the fact that it is intended
for present use at an altitude of 580 feet above sea level, which made
it possible to adjust the mercury level in the cistern so the
instrument the corrected pressure at sea level.
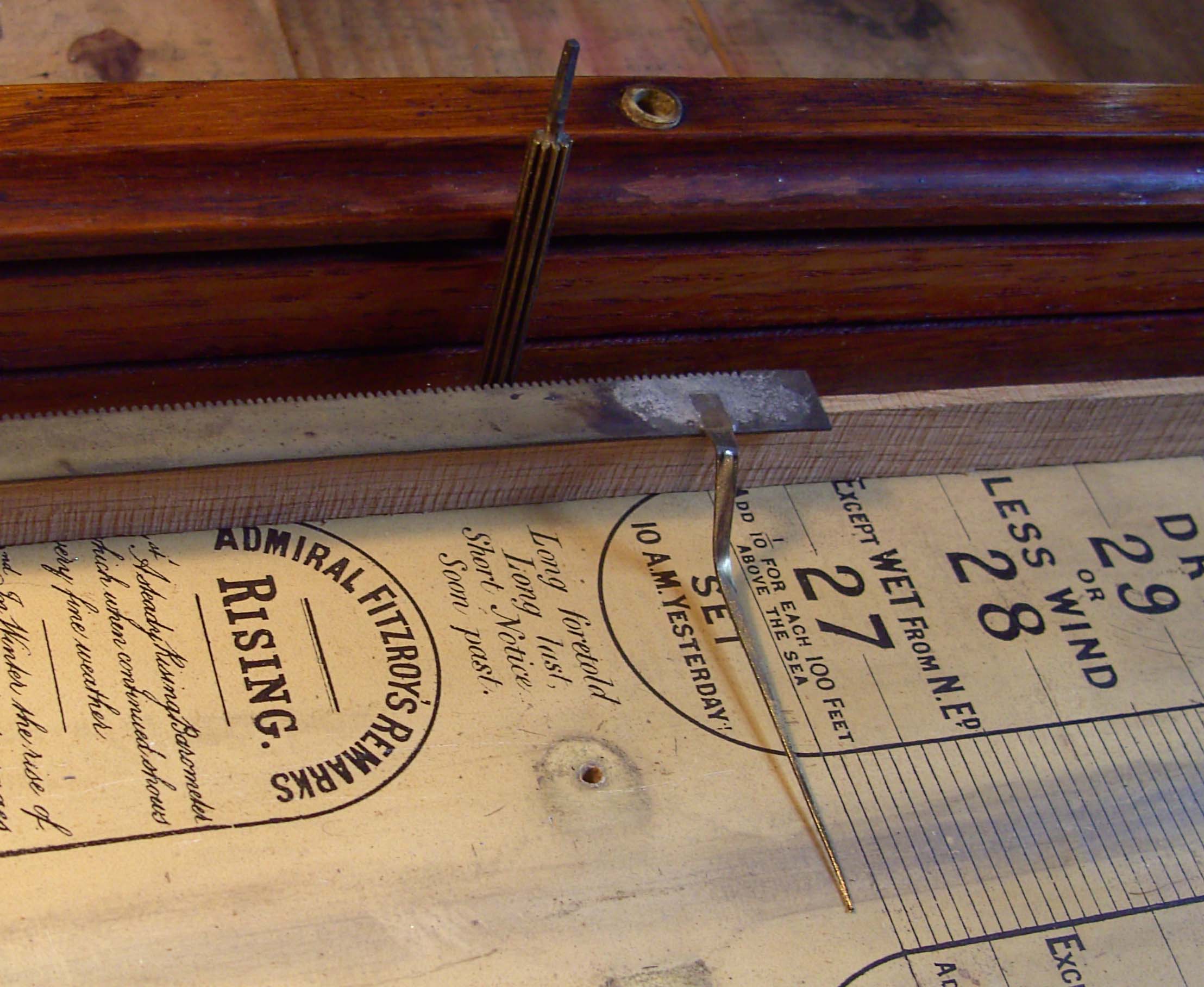
1. The
Case.
Figure 1 shows the overall instrument. The glass front slides in from
the bottom along grooves in the side plates and is retained by the base
of the instrument via two woodscrews securing the base to the case.
Removing these screws allows the base and glass to be removed. The
glass front had a semicircular top edge which fitted behind the carved
top. In the present case the glass was intact and so only required
cleaning. It was, however, thin Victorian glass and showed some minor
casting defects, so it was handled with considerable care.
The storm glass, which was empty of fluid, the thermometer which was
intact, and the barometer tube which was devoid of mercury were all
removed by carefully prising out the brass pins used to secure them.
These pins were polished and lacquered and set aside. With the
instrument components removed, the condition of the scales and interior
woodwork could be examined. The scale behind the stormglass had been
very adversely affected by the storm glass contents which had leaked
out all over it, reducing it to a friable, powdery condition from which
it could not be retrieved. It was carefully copied and a replacement
made. The main scale was in better condition and certainly worth
preserving, but was very darkened by age and not very easy to read. It
was resolved to leave it in place untouched, and to install a
replacement in such a way as to preserve the original (i.e. without
glue or any such thing) . It turned out rather easy to do this as there
were thin wooden fillets lining the frame which could be used
conveniently to secure the new scale.
2. The
Brass Components.
These comprised the pins, the saddles securing the glass parts, and the
index mechanisms on each side for recording readings. The pins and the
saddles were cleaned, polished and lacquered. The index mechanisms
needed some further work. Figure 3 shows how they function. The pinion,
shown removed from its slot in Fig 3, was a brass 10-leaf clock pinion,
filed to a 1.75mm square section at the upper end and turned to form a
blunt pivot at the lower end. It is introduced into its slot from the
rear of the instrument and retained there by a thin steel plate
perforated with a hole to fit the blunt pivot and having two other holes
through which screws held the plate to the case. In the subject
instrument, these plates had become completely corroded and had broken
with the consequent loss of the right hand pinion altogether. A
replacement was fashioned from 10-leaf pinion blank obtained from
skeletonclocks.
Two replacement steel plates were fashioned for the rear of the
instrument and these were made in 0.5mm stainless steel sheet to
prevent a repeat of the problem.
3. The
Storm Glass
The author is entirely unconvinced that the storm glass has any
reliable merit for weather observations and the most that can be said
for it is that it represents a very crude thermometer. However, it is a
historical component of the subject barometer and was restored using
the mixture advocated by Negretti and Zambra. This consists of 2.5gm of
Potassium Nitrate and 2.5 grams of Ammonium Chloride dissolved together
in 33ml distilled water. To this solution is carefully added a solution
of 10gms of natural Camphor in ethanol. According to some reports,
natural Camphor "works" better than synthetic because the latter
contains borneol which has an adverse effect. The mixing of the
solutions produces an immediate white precipitate of Camphor which
redissolves on warming. The storm glass tube shown in Figure 4, was
washed with detergent and filled with concentrated nitric acid and
allowed to stand overnight. It was then rinsed with distilled water,
allowed to drain and filled with the warmed storm glass mixture. It was
securely corked. As the mixture cooled a voluminous white precipitate
formed filling most of the tube and this slowly subsided to occupy less
than half the tube over a period of days.
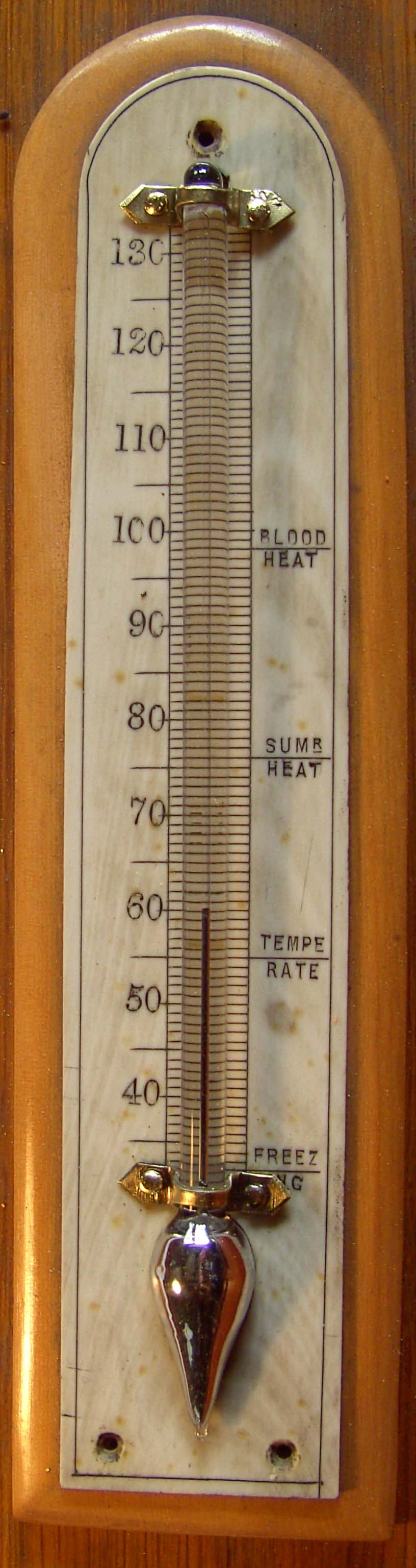
4. The
Thermometer
The Thermometer is shown in Figure 5. It is an attractive instrument,
mounted on hardwood (beech?) with a grained ivory scale The bulb is
pear shaped. Apart from cleaning and polishing the saddles, no other
restorative work was carried out.
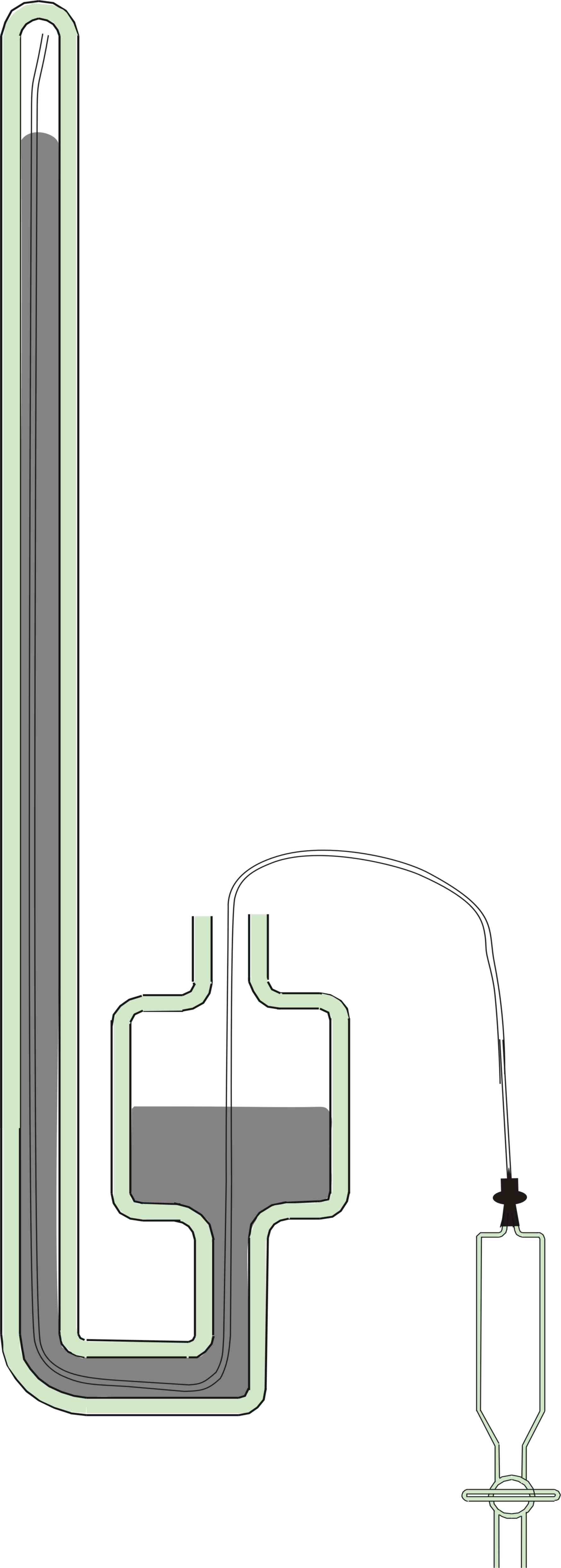
5. The
Barometer Tube
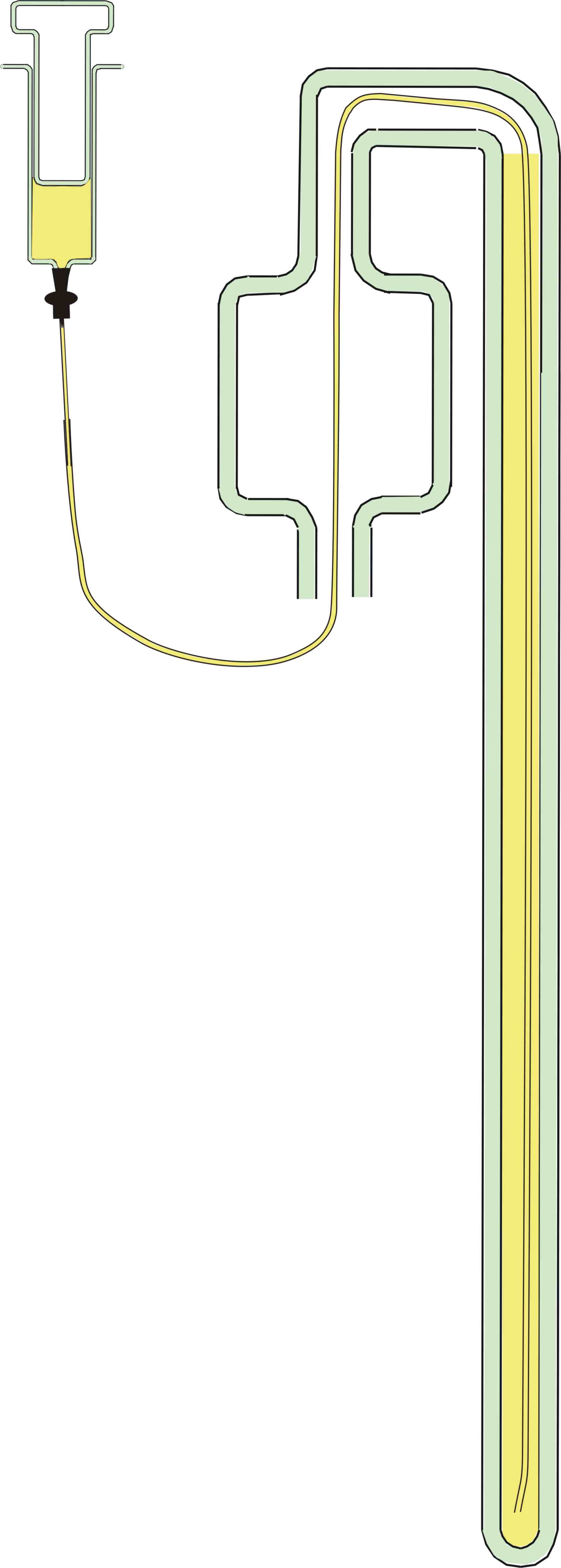
The Barometer tube was cleaned by filling it with concentrated nitric
acid to remove all Mercury and Mercury oxide residues using the
apparatus in Figure 6. A teflon tube 1mm ID and 1.6mm OD was threaded
to the far end of the barometer tube which was then clamped vertically
upside down. The teflon tube was attached to a glass syringe filled
with nitric acid and the barometer tube completely filled and left to
stand overnight. The nitric acid was then withdrawn and the barometer
tube set upright and a further quantity of nitric acid allowed to
remain for some hours in the cistern and U tube. The acid was then
withdrawn and the barometer tube washed thoroughly with water and then
rinsed with acetone. It was then pumped down to 50 microns of mercury
pressure by means of a good rotary vacuum pump and left under vacuum
for four hours.
Figure 7 shows the
apparatus used for filling the barometer tube with mercury. Again a
teflon tube 1mm ID & 1.6mm OD was used and threaded to the top
of the barometer tube, the latter clamped upright. A glass stopcock
sealed to a borosilicate syringe barrel was used to connect the free
end of the teflon tube to the vacuum line. Clean, doubly
vacuum-distilled mercury was poured into the cistern and the stopcock
opened to the vacuum line. The mercury was pulled up the barometer
tube to the barometric height. Thereupon the barometer was pumped
continually for 3 hours to achieve the best possible Torricellian
vacuum in the finished instrument. The stopcock was then closed and
immediately the teflon tube was withdrawn from the barometer tube,
filling the syringe with mercury in the process. The barometer tube was
checked for the correct height at the prevailing atmospheric pressure.
6.Assembly.
The installation of the barometer tube, the
securing of it with the brass saddles and the final installation of the
glass front and base was straightforward but great care was needed to
maintain sufficient vertical orientation of the barometer tube to
prevent any compromise of its vacuum. Rapid movements had to be avoided
to prevent the mercury striking the top of the barometer tube with any
significant force.
The barometer
reading was adjusted to that of sea level by adding or removing mercury
from the cistern.
The instrument was
provided with two-hole brass wall plate by which it could be suspended
on a wall a height convenient for reading the pressure.