ALL MATERIAL COPYRIGHT KEVIN SCOTT 2011. LINKS TO THIS SITE ARE WELCOME BUT DO NOT COPY MATERIAL FROM THIS SITE TO ANY OTHER WEBPAGE.
If you find this site useful, please support it by making a donation of $1 to help maintain and develop it. Click on the PAYPAL DONATE button to do this safely. But there is no obligation - please avail yourself of the information and facilities of the site at no charge.
As the European Union severely restricts the exporting of mercury, new means have to be found to preserve the craftsmanship and sophistication of traditional barometers in new, mercury-free instruments. The Sympiesometer or Air-barometer affords ample scope for elegance in design while providing improved precision and readability.
Most people are aware that the pressure exerted by the atmosphere is a primary indicator of the weather and is important for forecasting. The traditional way of measuring the atmospheric pressure was to balance it against a column of mercury in a tube. The tube is closed at the top end and dips in a mercury reservoir at the bottom and all the air is pumped out of it. As a result the mercury rises in the tube to a point where its weight ( or the pressure it exerts at the lower end of the tube) exactly matches the pressure exerted by the atmosphere. The space above the mercury in the tube is a vacuum. This arrangement is simply illustrated in figure 0. As the atmospheric pressure varies, the height of mercury needed to balance the pressure of the air also varies and so reading the height of the mercury column give the pressure exerted by the atmosphere.
Mercury is chosen because it is the liquid of highest density at room
temperature(13600kg/cubic metre) and has a very low vapour pressure.
(That is, it only evaporates to a very small extent into the vacuum
above the column). The high density means that the column can be
relatively short (about 760mm at sea level on average). No other liquid
can match the suitability of Mercury for barometry as is well
illustrated by describing a barometer made with water. The lower
density of water (1000kg/cubic metre) results in a barometer tube about
10 metres long, and the higher vapour pressure results in the water
boiling into the vacuum. Water barometers have been built: Gasparo
Berti, an Italian, was apparently the first to build such a water
barometer in about 1640 using a lead pipe bolted to the side of his
house and having a glass flask cemented to the top and a tank of water
at the bottom. It worked, but was not fully understood at the time.
By the mid-nineteenth century, Mercury had become established as the principal barometric fluid and the barometer developed to considerable degrees of precision and elegance. Fortin produced his famous barometer with the facility of setting precisely the lower level of mercury using an ivory pin to assist the observer in so doing. The height of the mercury was then measured precisely using Vernier scale. Other ingenious inventions allowed an iron float in the mercury to control a pointer on a circular scale. Elaborate and wonderfully engineered mechanisms were invented to record the atmospheric pressure from a mercury column on a paper chart, and a welter of simpler and beautifully crafted barometers adorned the walls of Victorian homes.
The fascination for monitoring the weather using a barometer has continued to the present, and the appreciation for finely crafted instruments is unabated. As most of these instruments centre on the use of mercury, it is a tragedy that the recent European Directive severely restricting the export of Mercury now threatens a distinguished and important area of scientific metrology. And this because of an irrational fear of the toxicity of mercury, and a disregard of its virtue and importance in scientific instruments. The scientific instrument manufacturers can only hope for wiser counsel to prevail in the future, and in the meantime, devote themselves to developing non-mecurial barometers to fill the void.
Happily enough there is plenty of space for creative innovation in the sphere of an alternative type of barometer, the so-called, air-barometer, or Sympiesometer.
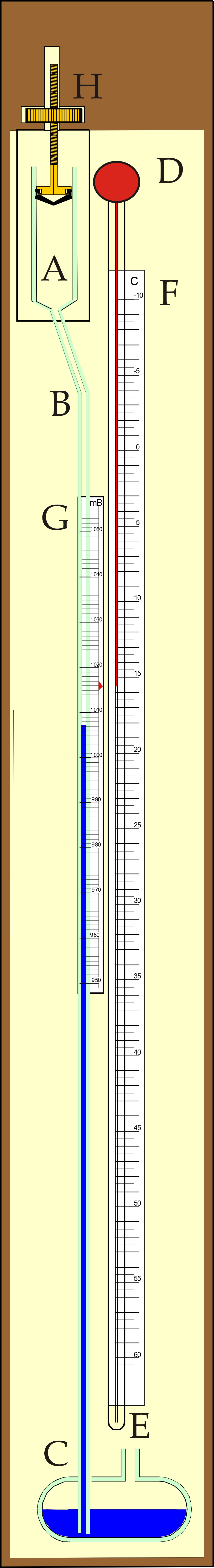
On January 2nd, 1667, an instrument was described to the Royal Society by Robert Hooke (1667), for measuring the barometric pressure, which could be used on board ship. Unfortunately no drawing survives but a demonstration was made of the instrument to the Royal Society and we have Hooke’s description from which it is clear that the instrument consisted of two parts: a vessel of air trapped over mercury such that changes in its volume could be observed and a spirit thermometer. These two parts were placed side by side so that volume changes in the gas arising from temperature changes could be resolved from those arising from pressure changes. In 1818, Alexander Adie (1818) published a Patent entitled "An improvement on the Air-Barometer" and with it, a diagram of the instrument. This is reproduced in fig 1. A bulb A, which Adie filled with hydrogen, is connected to the lower reservoir C, via the measurement tube. The instrument is provided with a thermometer, with a fixed scale OP, and a sliding scale MN calibrated for pressure. The thermometer is read and the sliding scale adjusted until its index is adjacent to the temperature on scale OP. The barometric pressure is then read by noting the position of the meniscus in the measurement tube against the sliding scale.
Adie’s instrument was very well received, not least by mariners in whose ships it was exhaustively tested. The Captain of one of the vessels in the Ross expedition to the Arctic commented: "The Sympiesometer is a most excellent instrument and shews the weather far better than the marine barometer. In short, the barometer is of no use compared to it…. In my opinion it surpasses the mercurial barometer as much as the barometer is superior to having none at all." (Quoted by Adie(1819))
Probably, there were two factors which prevented the Sympiesometer eclipsing the mercury barometer completely. The first was that the mercury barometer gave an immediate absolute value for the pressure. That is, by measuring the height of the mercury column, the atmospheric pressure was obtained without any further reference to any external standard. The second, and less serious factor, was that the temperature did not need to be measured for all normal purposes and no scale adjustments needed to be made to read the mercury barometer. By contrast, the Sympiesometer, although theoretically absolute, in practice needs to be set up using an independent measure of the atmospheric pressure. Again, the Sympiesometer needs to have the sliding scale so that the large effect of temperature can be adequately compensated.
The modern availability of
atmospheric pressure data to enable easy set up of the Sympiesometer
removes the chief objection to its use. Moreover, the Sympiesometer has
two great advantages over mercury barometers. The first is the greatly
enhanced readability of the instrument. By appropriate choice of
dimensions, the scale of the instrument can be set to any convenient
sensitivity. In the instruments described below, the sensitivities were
adjusted to be 0.5mb/mm and 1mB/mm, respectively. The second, important
in modern context, is that the Sympiesometer can readily be adjusted to
read the atmospheric pressure pertaining at sea level, even when the
instrument is operated at an elevated location.
The rate of the thermometer is adjusted so that it is equal to the thermal expansion of the gas in A. This makes reading the atmospheric pressure a very simple matter. The sliding scale is adjusted until the index is opposite the thermometer thread, and the atmospheric pressure read from the meniscus in the measuring tube against the pressure graduations on the sliding scale.
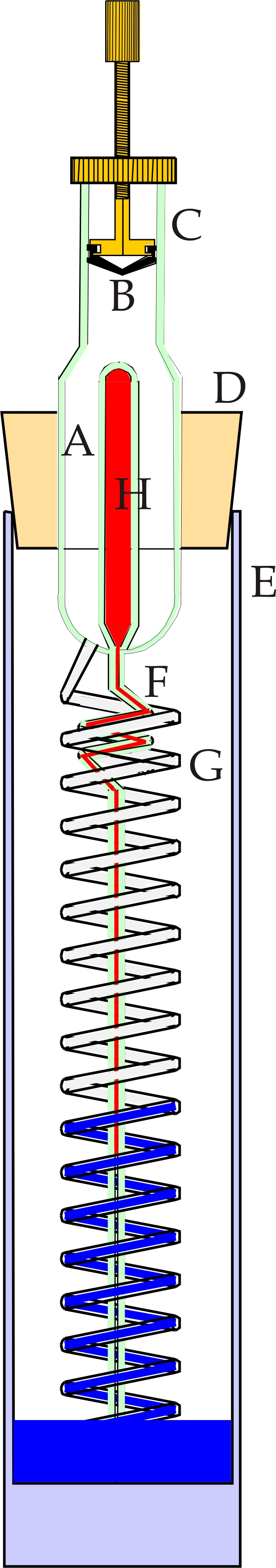
An alternative design of sympiesometer is shown in figure 3, operating on the same principles as the foregoing but capable of a readability and precision of 1mB but without any printed scales. The Captive Gas Volume A is fitted with an adjustable piston B sliding in a precision bore tube C. A thermometer bulb H is sealed in to A and leads to a capillary thermometer stem F. The Captive gas volume A is connected to glass helix G which is carefully wound with a uniform pitch of 10 mm. The whole is mounted via cork stopper into glass cylinder E. A slot (not shown) machined in cork D allows the atmospheric pressure to reach the interior of E. A quantity of barometric fluid is placed in E and the cork and glass assembly replaced. By adjustment of piston B, the fluid level can be set.
The height of the fluid level is related to the atmospheric pressure by a very nearly linear relationship. The dimensions of the components were calculated and adjusted to give precisely a sensitivity 10 hPa per vertical centimetre, i.e. 10 hPa per turn of the glass helix G. The thermometer dimensions were adjusted to exhibit the same rate with temperature as the gas expansion rate linearly along the axis of the helix. At a fixed pressure, there is, therefore, the same linear displacement between the end of the thermometer thread and the level of barometric fluid in the helix G at all temperatures in the working range of the instrument.
If the whole instrument is rotated
about its vertical axis while the eye remains level with the end of the
thermometer thread, a point will be reached when the thread is exactly
level with the top of a turn of the helix G at a point nearest to the
eye. This set point is chosen to represent 1000 hPa. The instrument is
set up so that if the level of the barometric fluid lies below the set
point, the pressure prevailing is less than 1000hPa, and if above, then
the pressure is greater than 1000 hPa. This means that each full turn
of the helix represents 10 hPa below or above the 1000 hPa set point.
This makes the instrument very easy to read. It is first rotated to
locate the set point and the level of the fluid noted in full turns and
a fraction of a turn above or below the set point. This gives the
atmospheric pressure directly.
Tests on the two instruments described here showed how very justified was the enthusiasm which greeted the first Sympiesometers when they made their debut more than 200 years ago.
Figure 4 shows the barometric trace for a period of 1345 minutes from 9:25am on October 22nd 2008, in the village of Stow, Selkirkshire Scotland as measured by an electronic standard (Sensortechnics,type 144SC0811BARO. (Sensortechnics, 2008)). Superimposed on the plot are the measurements taken from the wall-mounted Sympiesometer. The standard deviation between the two sets of data amounts to 0.16mB The instrument thus exhibits a superior precision to that often shown by mercury barometers.
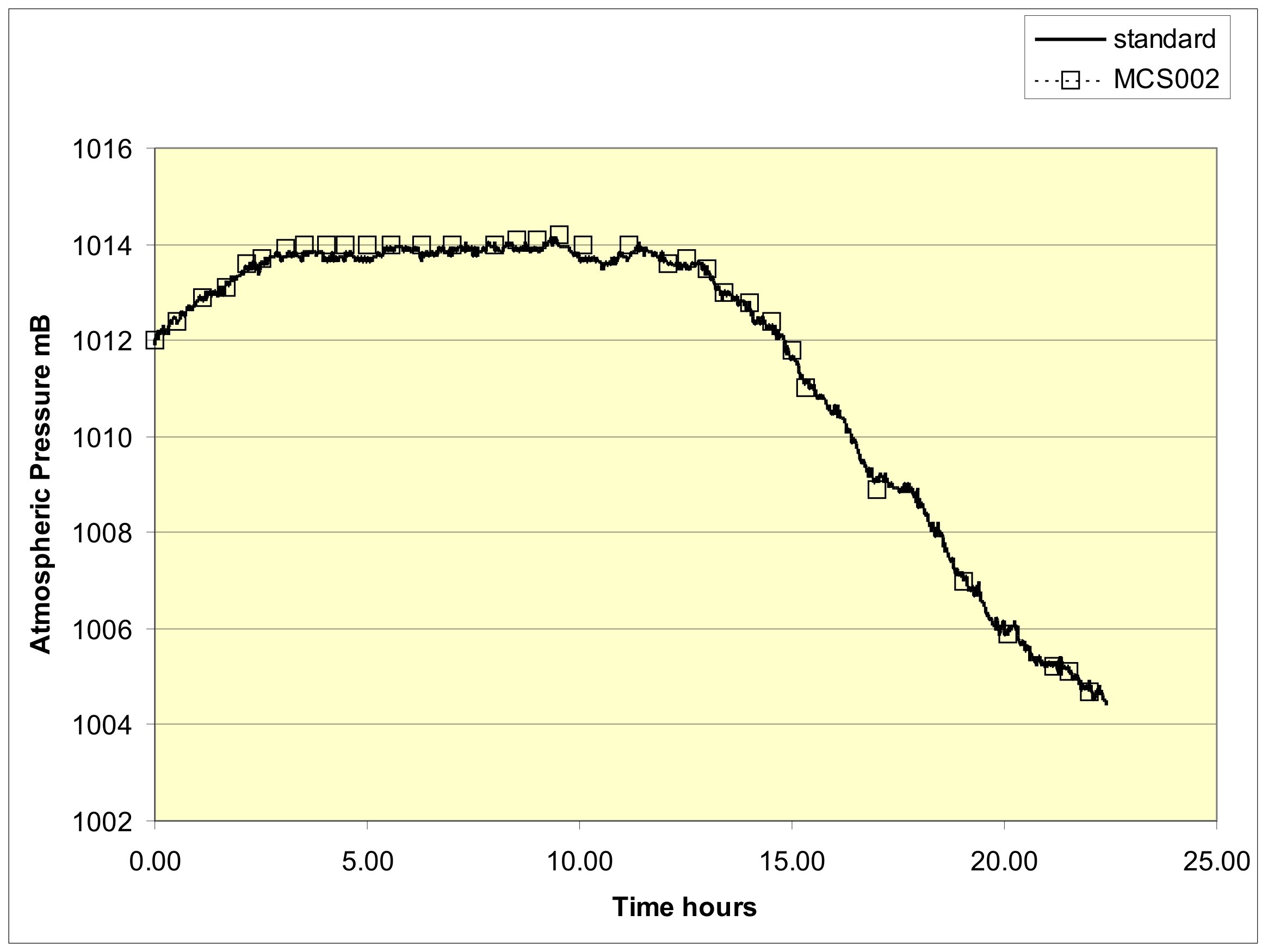
Figure 4, A barometric trace obtained from readings taken from a Sympiesometer type MCS002, compared to a standard.
A comparison between the wall-mounted and table-top Sympiesometers is shown in Figure 5. This shows the barometric trace for a period of 2100 minutes from 8:45am on October 19th 2008, also in the village of Stow. The standard deviation between these to sets of data is 0.9mB and this can be taken as a measure of the accuracy of the TTS002 instrument in the range examined.
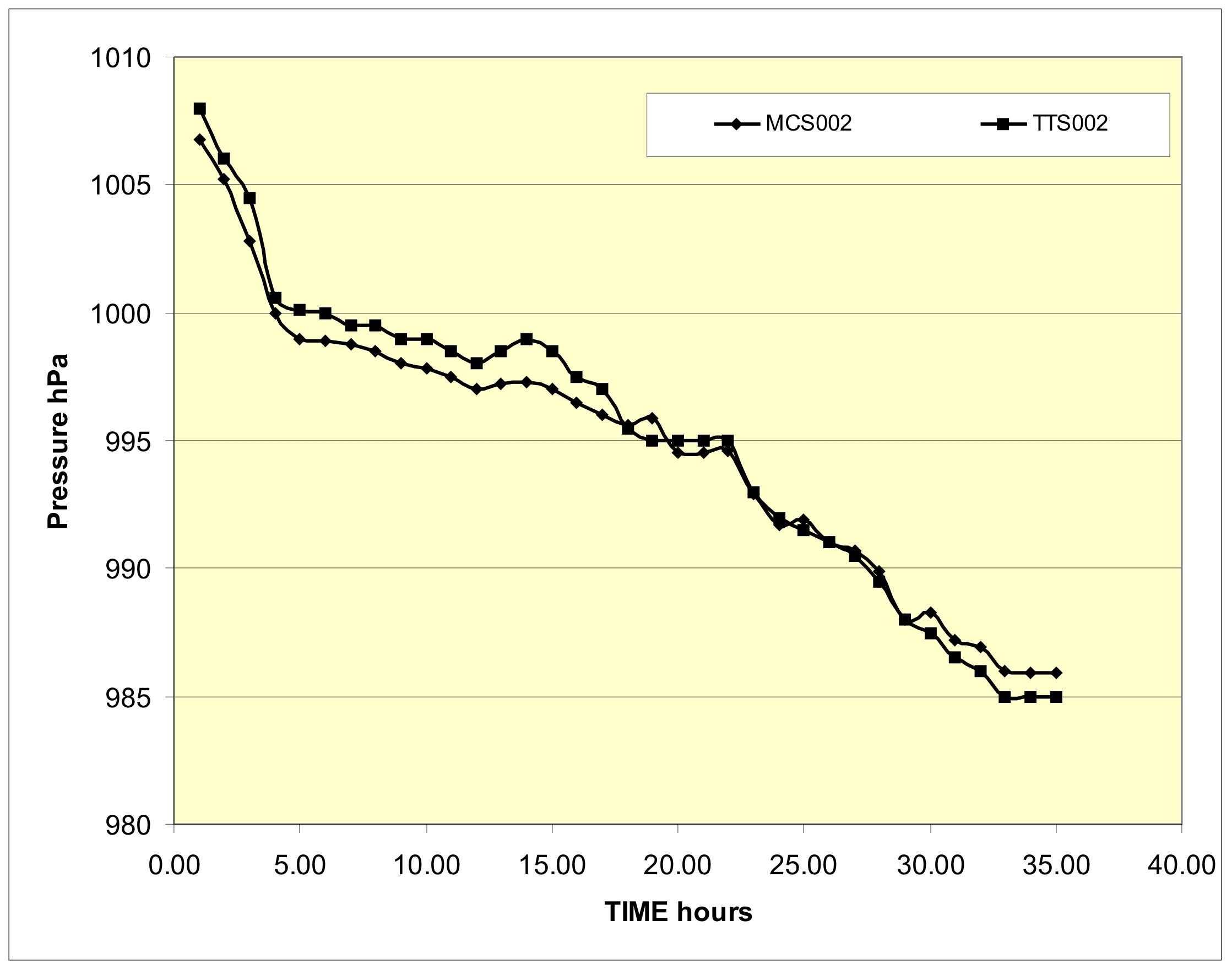
Figure 5 The performance of the TTS002 compared with MCS002
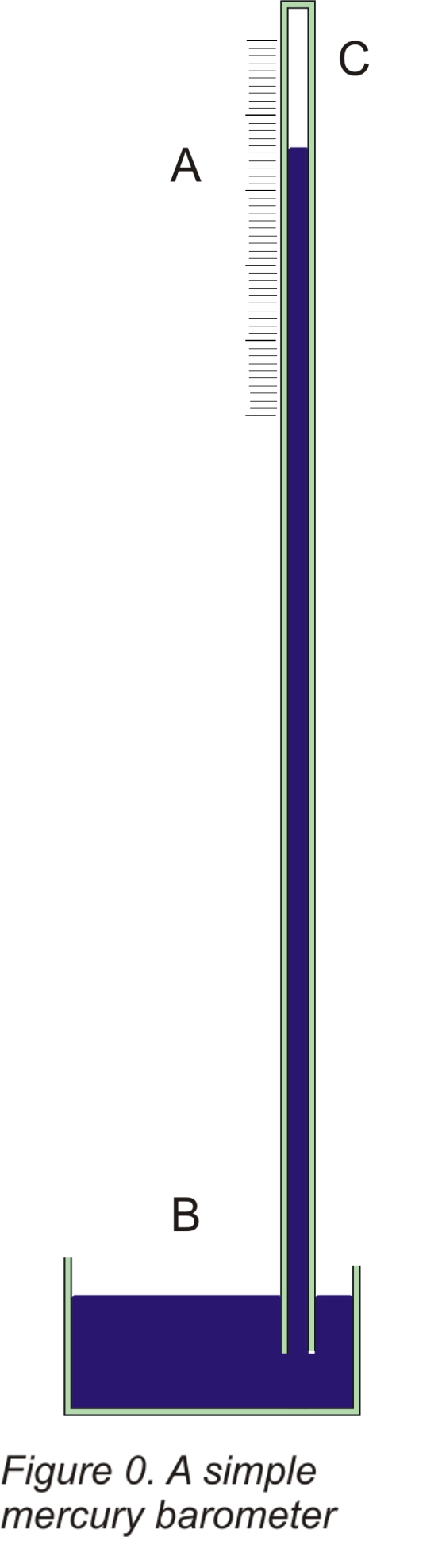
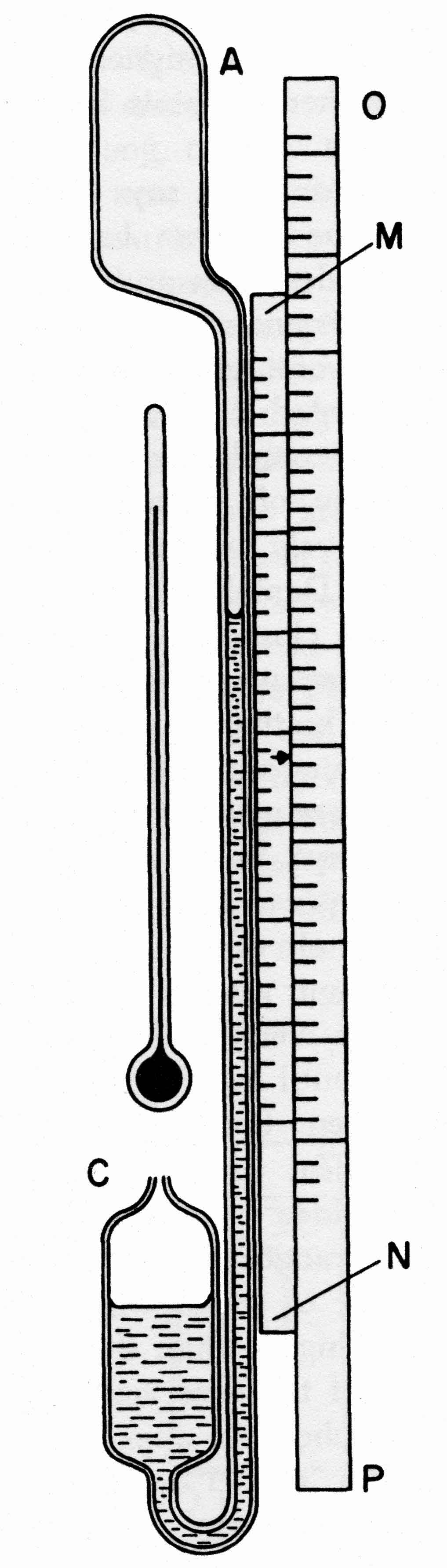
Figure 1. The original sketch of Adie's Sympiesometer from his patent of 1818.