ALL MATERIAL COPYRIGHT KEVIN SCOTT 2011. LINKS TO THIS SITE ARE WELCOME BUT DO NOT COPY MATERIAL FROM THIS SITE TO ANY OTHER WEBPAGE.
If you find this site useful, please support it by making a donation of $1 to help maintain and develop it. Click on the PAYPAL DONATE button to do this safely. But there is no obligation - please avail yourself of the information and facilities of the site at no charge.
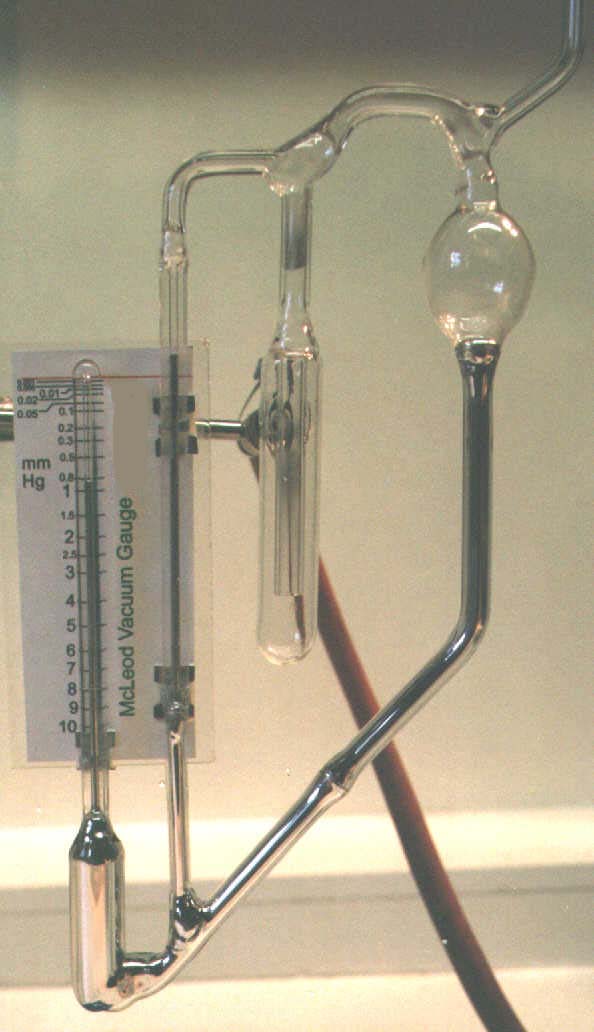
A simple McLeod Gauge similar to that depicted right, can readily be constructed in the laboratory and will often prove an asset where pressures in the range of 0.001mm Hg to 10 mm Hg need to be measured with reasonable accuracy. The principle of operation is simple: The gauge is exposed to the pressure to be measured and the instrument rotated to the horizontal position shown in the plate below:
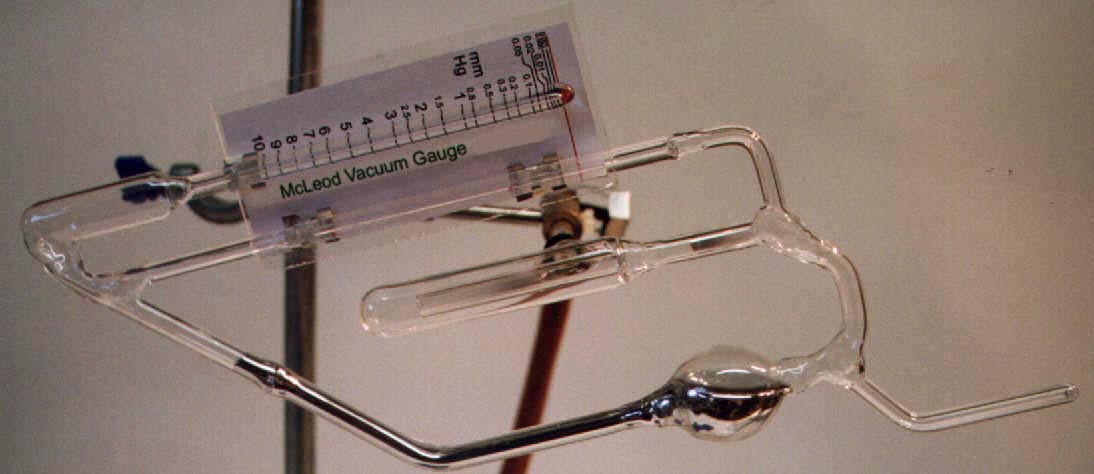
A short period is allowed to elapse to allow the gas in the gauge reservoir to come to the pressure to be measured, whereupon the instrument is restored to its vertical position such that the mercury meniscus in the right hand capillary is level with the red index line on the scale. The pressure is read from the position of the left-hand meniscus against the calibrated scale. During the operation of the instrument the gas at the system pressure in the lower left reservoir is compressed by the mercury into the left hand closed capillary. The instrument assumes Boyle's Law to hold for gases at low pressures, and to calibrate it is only necessary to measure the volume of the reservoir and the bore of the closed capillary. Various designs of the instrument can accommodate different reservoir volumes, the larger giving a lower limit to the measurable pressure.
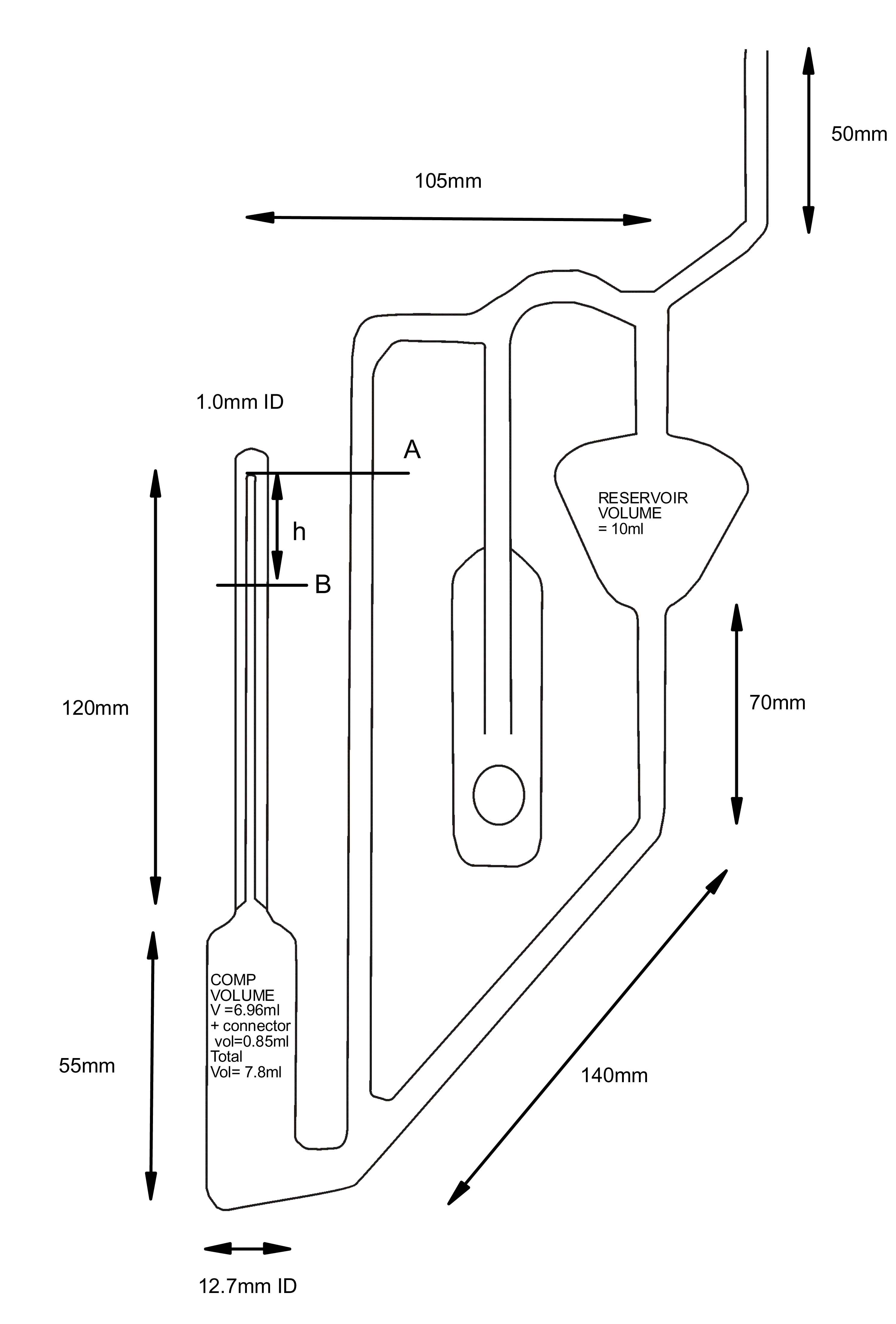
Suitable dimensions of a laboratory instrument are shown in the figure. The compression volume is carefully measured as it controls the scale calibrations (see below) The Upper right reservoir is less critical but the instrument must have sufficient mercury introduced into it to enable the level in the right hand vertical tube to reach level A in the course of measurement for all pressures in the range.
Specifications for the prototype to enable the scale to be constructed are given below, right, and the resulting scale is shown below left:
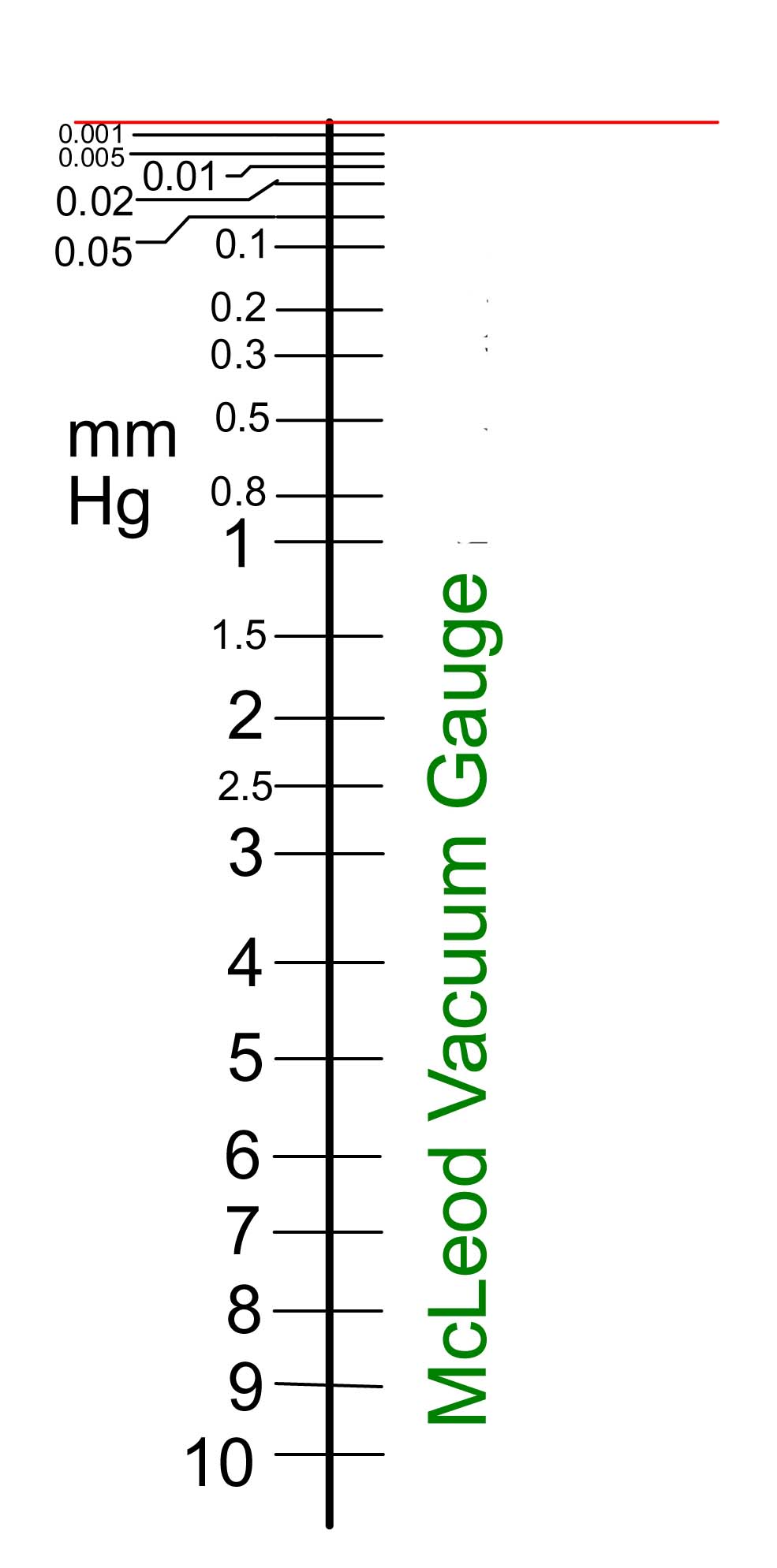
 The precision to which a pressure can be measured clearly falls with falling pressure, although for most purposes, measurements of low pressures to within an order of magnitude is often sufficient. To improve the low pressure range and precision it is necessary to increase the compression volume and this is not practicable for an instrument such as this where the rotation of the instrument is required for making measurements.
The precision to which a pressure can be measured clearly falls with falling pressure, although for most purposes, measurements of low pressures to within an order of magnitude is often sufficient. To improve the low pressure range and precision it is necessary to increase the compression volume and this is not practicable for an instrument such as this where the rotation of the instrument is required for making measurements.
As with all instruments employing mercury, care needs to be taken in the operation of the McLeod Vacuum Gauge. In particular, the following should be observed:
1) Rotate the instrument slowly when required to make a measurement.
2) Do not rotate the instrument beyond its required angles for correct operation.
3) Leave the instrument in the horizontal position when no measurements are to be taken.
4) Pump the instrument down in the horizontal position slowly, and bring the instrument up to atmospheric pressure when required also slowly.
5) Provide a tray or trough below the instrument to catch any spilled mercury should a breakage occur.