ALL MATERIAL COPYRIGHT KEVIN SCOTT 2011. LINKS TO THIS SITE ARE WELCOME BUT DO NOT COPY MATERIAL FROM THIS SITE TO ANY OTHER WEBPAGE.
If you find this site useful, please support it by making a donation of $1 to help maintain and develop it. Click on the PAYPAL DONATE button to do this safely. But there is no obligation - please avail yourself of the information and facilities of the site at no charge.
One of the disadvantages of pure silver chloride as a cement is its high melting point of about 450 deg C. Mixing silver chloride with lead chloride or thallous chloride reduces the melting point and possibly reduces the coefficient of linear expansion. The Phase diagram for the AgCl/PbCl2 system is plotted in the figure ( after Trent & Welsh 1966)
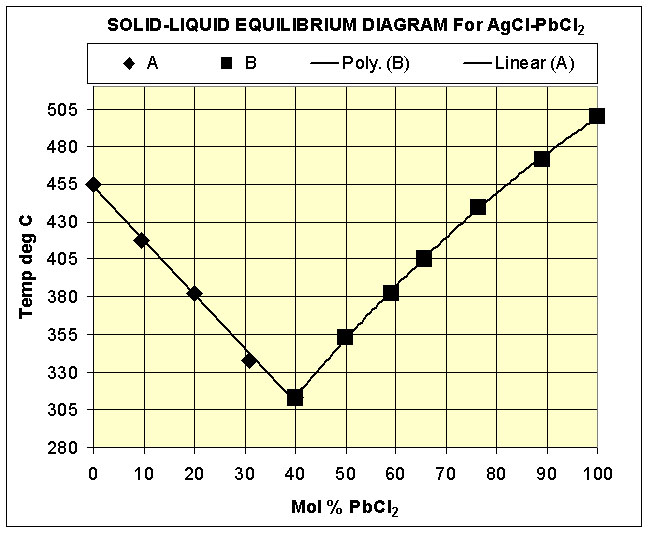
The eutectic at about 40% Mol ratio of lead chloride melts at about 310 deg C. A small quantity of this eutectic mixture can be prepared by taking 5 gms of silver nitrate crystals and 6.5 gms of lead nitrate crystals and dissolving them together in 5o ml of distilled water. When the nitrates have fully dissolved, 15ml of concentrated hydrochloric acid is added, resulting in a white precipitate of the mixed chlorides. The mixture is allowed to stand in the dark overnight and then filtered and the precipitated washed with a little distilled water and dried. The resultant dried material is ground to a fine powder in a mortar.