ALL MATERIAL COPYRIGHT KEVIN SCOTT 2011. LINKS TO THIS SITE ARE WELCOME BUT DO NOT COPY MATERIAL FROM THIS SITE TO ANY OTHER WEBPAGE.
If you find this site useful, please support it by making a donation of $1 to help maintain and develop it. Click on the PAYPAL DONATE button to do this safely. But there is no obligation - please avail yourself of the information and facilities of the site at no charge.

Chemical microscopy involves a wide variety of specimen types: crystalline materials, precipitates of all kinds, opaque solids, minerals, plastics and colloids. Correspondingly this wide range has prompted the use of many different types of microscope of which the following are examples:
(i) Metallurgical microscopes.
(ii) Polarising microscopes.
(iii) The ultramicroscope.
(iv) The phase contrast microscope.
On this page, the first two instruments in the above list are described because they open up the widest range of possibilities for the chemical microscopist.
The photograph to the left shows a Watson Barnet Standard Metallurgical Microscope. The feature which distinguishes it from standard instruments is the vertical illuminator installed in the microscope tube to enable opaque obects to be illuminated through the microscope objective.
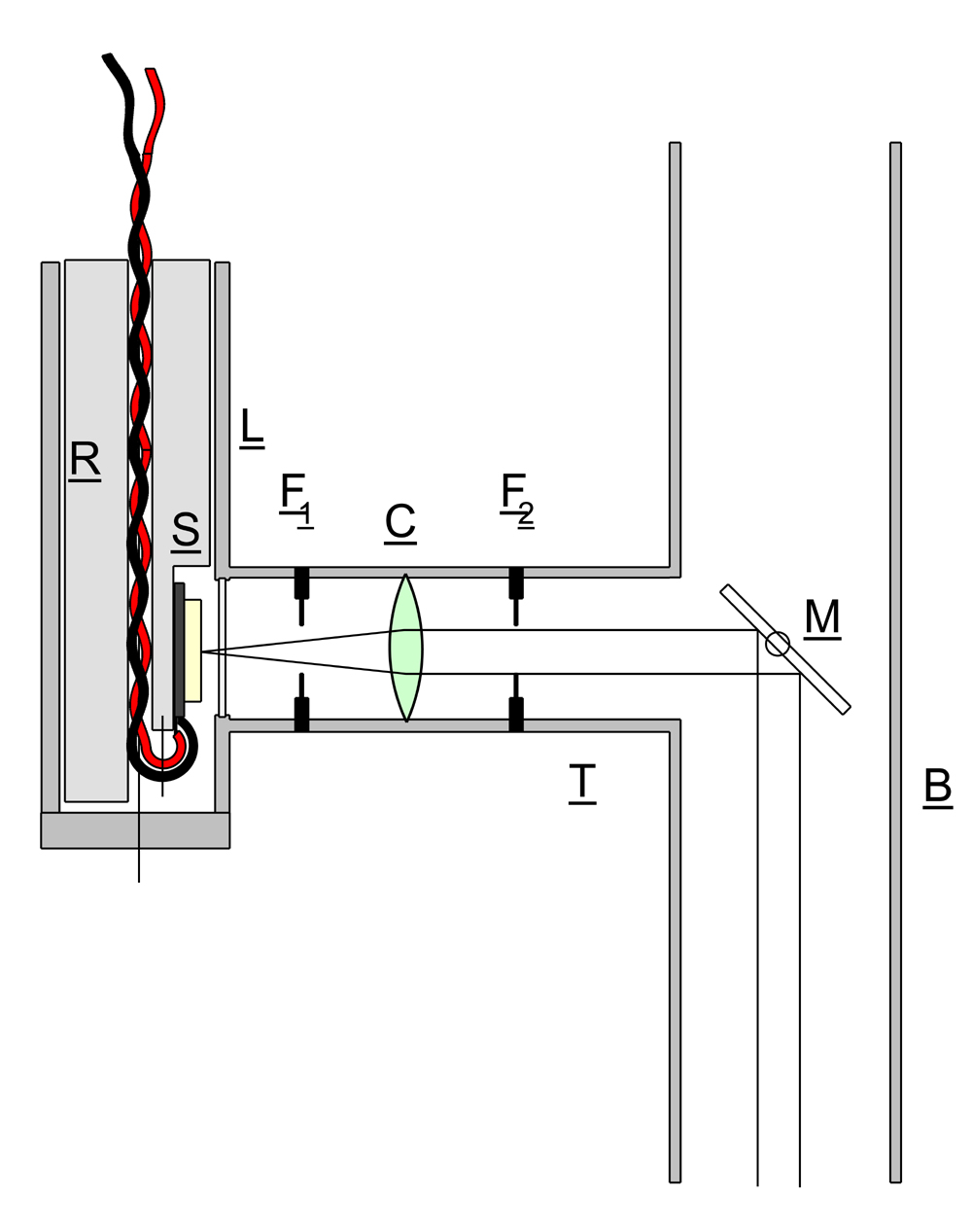
In the instrument described, the vertical illuminator took the from shown in the sketch. Side tube T is mounted at right angles to the microscope barrel B and carries a lens C, and two Iris diaphragms F1 and F2.
Lamphousing L originally contained tungsten lamp, but this obsolete item was replaced with a white LED lamp S (0.5W) mounted on a machined aluminium insert R and powered by a dc current source. A variable voltage supply was used 0-12V and a 50 ohm 25W resistor wired in series with the LED. This gave an easily controlled variation in intensity of light from the LED across the whole range of its capability. A polished glass disk M was installed in the microscope barrel to reflect the collimated beam downwards to illuminate the specimen. Disk M could be adjusted to the correct orientation by means of a knurled wheel on the outside of the barrel. Diaphragm F1 could be used to make adjustments to the light intensity, while F2 controlled the diameter of the beam which had to be kept within the available diameter of the reflector M to avoid spurious light reflections from the inside of the barrel.
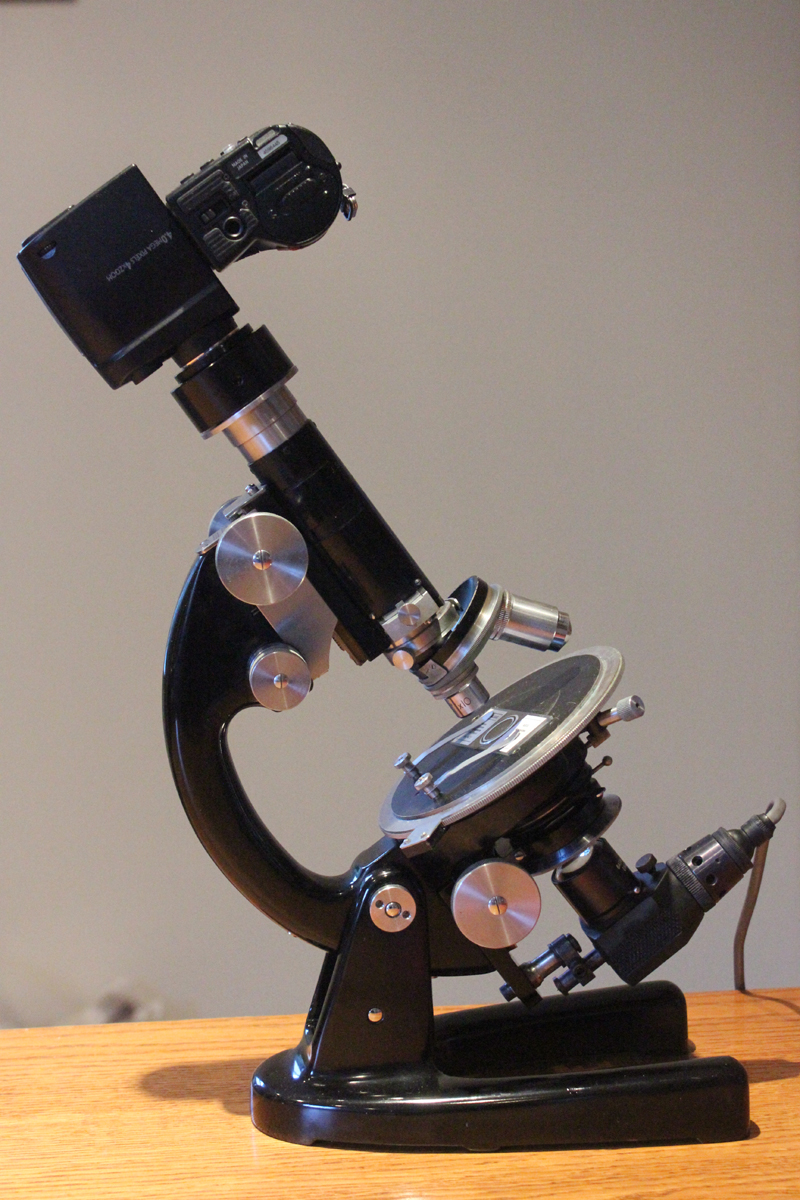
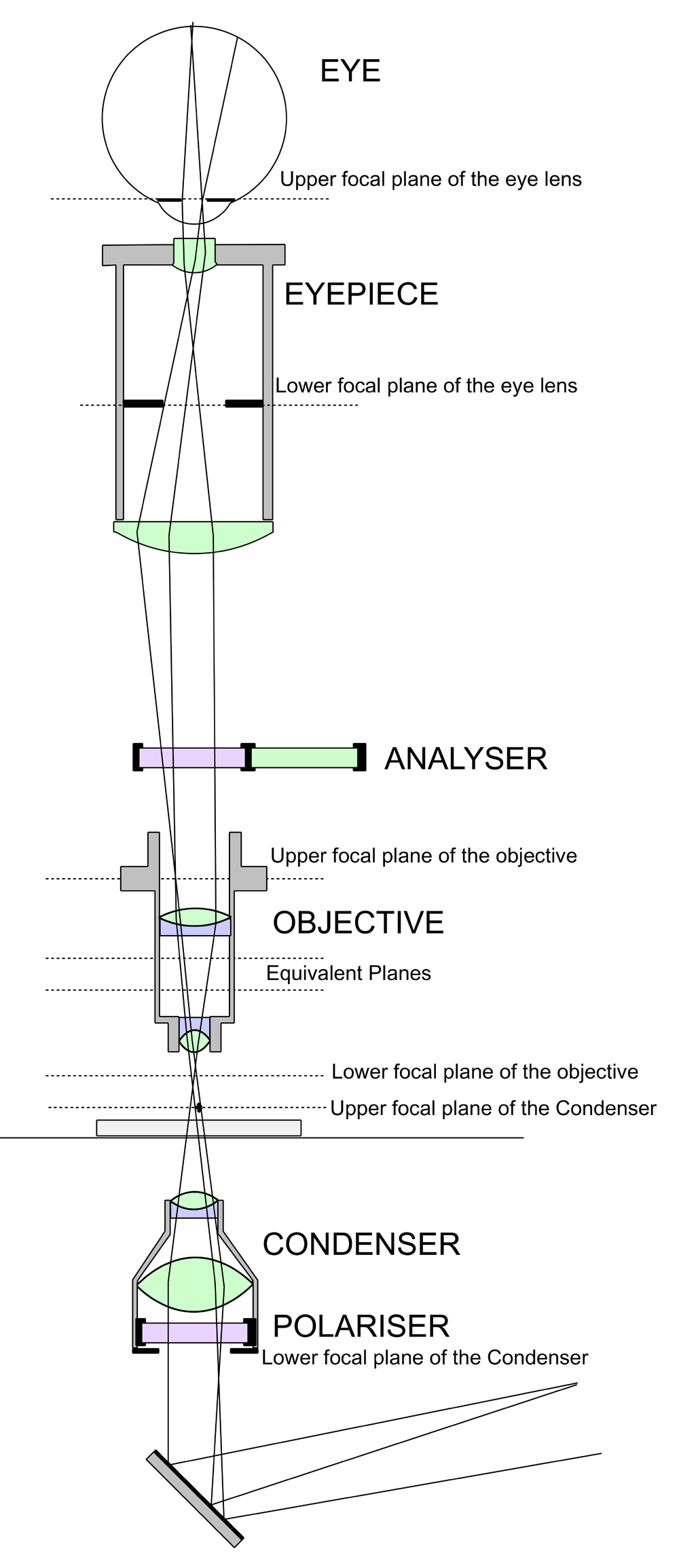
One of the greatest assets to the chemical microscopist must be the polarising microscope. Pictured to the right is a Swift Model P instrument, the optical arrangement of which is shown in the sketch on the left. The basic configuration is the same as a standard microscope but with some important additions. Light entering the condenser passes first through a nicol prism which provides plane polarised light, the plane of which is rotated by means of a calibrated ring on the polariser mounting. A similar nicol prism serves as an analyser and is installed on a sliding mount which can at will be inserted in the optical path of the microscope. The eyepiece is equipped with crosshairs and a facility for setting them at 45° to their normal position. The stage is circular and rotatable with a vernier scale calibrated in degrees.

The polarising microscope provides a powerful means of examining the very wide range of materials which exhibit optical anisotropy and it may be useful to give a short account of the phenomenon here with particular reference to the types of measurements of which a polarising microscope is capable.
In general, the optical properties of a transparent solid are governed by the arrangement of atoms, molecules or ions in the lattice. Except in the case of cubic crystals, the configuration of these species in the crystal means that the properties exhibited along one axis will not be the same as those along another. In the simplest case, along two axes of a crystal (referred to as the optic axes) plane polarised light of a particular orientation will pass through unchanged. These two axes differ in that the panes of polarised light which they transmit are at right angles to each other. The refractive indices along the two optic axes are also different which means that the polarised beams travel at different speeds along their respective axes.
If plane polarised light is passed through the crystal in a direction other than along one of optic axes, the polarisation is resolved into to components at right-angles corresponding to the properties of the two optic axes. These two components will travel at different speeds through the crystal and therefore their phase relationship will vary continually. The light beam will have a polarisation which results from the vector sum of the two orthogonal components. Since the two beams are moving at different speeds, the phase relationship continually changes along the path of the beam, so that the vector sum of the components will vary accordingly. At a certain distance along the path, the two polarised components with be out of phase by 90° which will result in a circularly polarised beam. (This is the quarter wave distance) After a similar distance again, the beam will be plane polarised at 90° to the incoming beam. ( The total distance in this case being termed the half-wave distance)
All this is exactly true for monochromatic light, but since the refractive indices vary with wavelength, if white light is used, a given half-wave distance will only be correct for one colour. So that a crystal of thickness required for half-wave performance with, say, blue light, mounted between crossed nicol prisms, will result in no blue light reaching the observer. But red light will be transmitted. It is possible to create colour filters using this technique.
The polarising microscope can be used to detect anisotropy, determine the directions of the optic axes and investage the relationship between the two refractive indices exhibited by a particular crystal. The effectiveness of these investigations depends upon the sample preparation, particularly the quality of the crystals produced for study.